
Karen Buchmueller
Associate Professor, Chemistry
- Email: karen.buchmueller@furman.edu
- Phone: 864.294.2683
- Office: Plyler 236D, Townes Science Center
Karen Buchmueller graduated from The College of Wooster with a B.A. in chemistry and a minor in biology. She then pursued her Ph.D. in chemistry, with a specialization in biochemistry at University of North Carolina, Chapel Hill. She worked in the lab of Dr. Kevin M. Weeks to characterize the folding of functional RNA. She also had her first experience teaching biochemistry as a GAANN fellow at UNC and realized her passion for teaching. After completing her doctorate, she was a Dreyfus Postdoctoral Fellowship at Furman University working with Dr. Moses Lee and Dr. W. David Wilson (Georgia State University) on the biophysical characterization of novel polyamides that selectively bind DNA.
After completing her postdoc, Dr. Buchmueller was hired as an Assistant Professor at Wake Forest University in the Chemistry Department. Later, she returned to Furman in 2007 as the department's biochemist. In 2014, she was named the Henry Keith and Ellen Hard Townes Associate Professor. She is also the co-director of the NSF-REU program in the Chemistry Department at Furman University.
Education
- Ph.D., University of North Carolina at Chapel Hill
- B.A., College of Wooster
Research
The Buchmueller research group is investigating how proteins interact with DNA. We are particularly interested in the HMGA family of proteins, which are linked to cancer metastasis. These proteins are intrinsically disordered and have been difficult to characterize, but there are a variety of biophysical techniques that have provided insight into the molecular nature of how these proteins interact with DNA. It has been long known that HMGA proteins bind the minor groove of AT rich DNA using AT hook motifs. Much of our research utilizes peptides that mimic AT hook motifs, so we can use biophysical techniques to understand the interactions between these AT hooks and DNA. Specifically, we have used isothermal titration microcalorimetry (ITC) to characterize the effects of salt, temperature and DNA sequence on AT hook interactions with DNA.
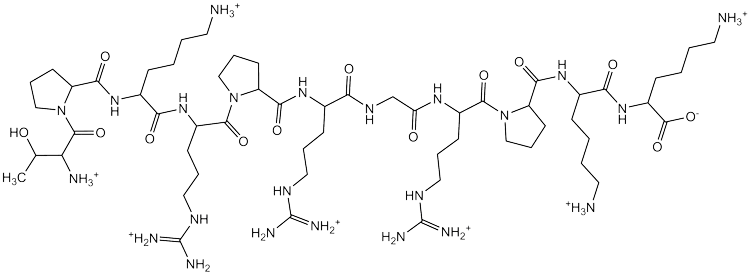
For a variety of reasons, including the disordered structure of these proteins, it is difficult to develop therapeutic targets against HMGA proteins. We seek to better characterize the interactions with DNA so that a therapeutic model can be developed. For example, we are looking at the competition between small molecules and the peptide mimics at the molecular level to gain insight.
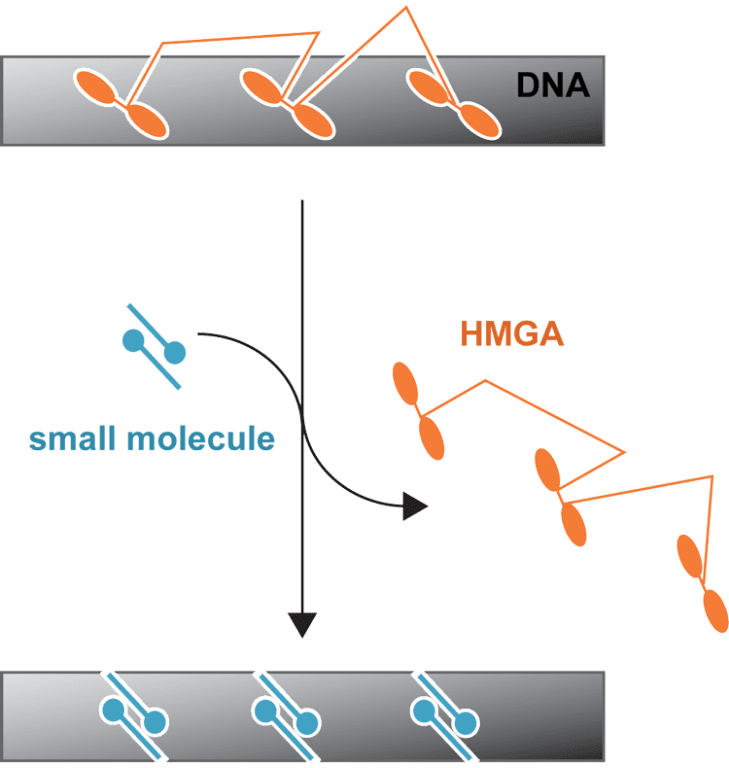
Students in the Buchmueller lab will use a variety of biophysical, bioanalytical and molecular biology techniques to understand how small molecules and/or proteins interact with DNA.
-
Smith, A. E.; Buchmueller, K. L. Molecular basis for the inhibition of HMGA1 proteins by distamycin A. Biochemistry 2011, 50, 8107-16.
-
Brown, T.; Taherbhai, Z. T.; Sexton, J. S.; Sutterfield, A.; Turlington, M.; Jones, J. B.; Stallings, L.; Stewart, M.; Buchmueller, K. L.; Mackay, H.; O'Hare, C.; Kluza, J.; Nguyen, B.; Wilson, D.; Lee, M.; Hartley, J. A. Synthesis and biophysical evaluation of minor-groove binding C-terminus modified pyrrole and imidazole triamide analogs of distamycin. Bioorg. Med. Chem. 2007, 15, 474-83.
-
Buchmueller, K. L.; Bailey, S. L.; Matthews, D. A.; Taherbhai, Z. T.; Register, J. K.; Davis, Z. S.; Bruce, C. D.; O'Hare, C.; Hartley, J. A.; Lee, M. Physical and structural basis for the strong interactions of the -ImPy- central pairing motif in the polyamide f-ImPyIm. Biochemistry 2006, 45, 13551-65.
-
Flores, L. V.; Staples, A. M.; Mackay, H.; Howard, C. M.; Uthe, P. B.; Sexton, J. S.; Buchmueller, K. L.; Wilson, W. D.; O'Hare, C.; Kluza, J.; Hochhauser, D.; Hartley, J. A.; Lee, M. Synthesis and evaluation of an intercalator-polyamide hairpin designed to target the inverted CCAAT box 2 in the topoisomerase IIα promoter. ChemBioChem 2006, 7, 1722-9.
-
Buchmueller, K. L.; Horick, S. M.; Howard, C. M.; Uthe, P. B.; Staples, A. M.; Bailey, S. L.; Le, N. M.; Cox, K. K.; Henry, J. A.; Lee, M. Recognition of Specific DNA Sequences by Stacked Pyrrole- and Imidazole- Containing Polyamides: An Efficient Screening Method Based on Competitive Dialysis. Lett. Drug Des. Discovery 2005, 2, 137-142.
-
Buchmueller, K. L.; Staples, A. M.; Howard, C. M.; Horick, S. M.; Uthe, P. B.; Le, N. M.; Cox, K. K.; Nguyen, B.; Pacheco, K. A. O.; Wilson, W. D.; Lee, M. Extending the language of DNA molecular recognition by polyamides: unexpected influence of imidazole and pyrrole arrangement on binding affinity and specificity. J. Am. Chem. Soc. 2005, 127, 742-50.
-
Buchmueller, K. L.; Staples, A. M.; Uthe, P. B.; Howard, C. M.; Pacheco, K. A. O.; Cox, K. K.; Henry, J. A.; Bailey, S. L.; Horick, S. M.; Nguyen, B.; Wilson, W. D.; Lee, M. Molecular recognition of DNA base pairs by the formamido/pyrrole and formamido/imidazole pairings in stacked polyamides. Nucleic Acids Res. 2005, 33, 912-21.
-
Buchmueller, K. L.; Taherbhai, Z. T.; Howard, C. M.; Bailey, S. L.; Nguyen, B.; O'Hare, C.; Hochhauser, D.; Hartley, J. A.; Wilson, W. D.; Lee, M. Design of a Hairpin Polyamide, ZT65B, for Targeting the Inverted CCAAT Box (ICB) site in the Multidrug rRsistant (MDR1) Gene. ChemBioChem 2005, 6, 2305-11.
-
Uthe, P. B.; Staples, A. M.; Turlington, M.; Jones, J. B.; Blackmon, K. N.; Bailey, S. L.; Buchmueller, K. L.; Lee, M. Novel Picolinic Acid-containing Pyrrole-Imidazole Polyamides: Synthesis and T-G Mismatched Base Pair Recognition. Heterocycl. Commun. 2005, 11, 163-166.
-
Henry, J. A.; Le, N. M.; Nguyen, B.; Howard, C. M.; Bailey, S. L.; Horick, S. M.; Buchmueller, K. L.; Kotecha, M.; Hochhauser, D.; Hartley, J. A.; Wilson, W. D.; Lee, M. Targeting the inverted CCAAT box 2 in the topoisomerase IIα promoter by JH-37, an imidazole-pyrrole polyamide hairpin: design, synthesis, molecular biology, and biophysical studies. Biochemistry 2004, 43, 12249-57.