John Wheeler
Associate Provost for Integrative Science; Professor of Chemistry
- Email: john.wheeler@furman.edu
- Phone: 864.294.3371
- Office: Townes Science Center 071
John Wheeler is the Associate Provost for Integrative Science at Furman and has been a faculty member in the Chemistry Department since 1991. A recipient of the South Carolina Governor’s Award for Excellence in Scientific Research, he has also received Furman’s Meritorious Advising Award and the Henry Keith and Ellen Hard Townes Professorship. He has mentored more than 130 undergraduates and seventeen M.S. students conducting research in bioanalytical chemistry, publishing thirty articles at Furman as supported through grants from NSF, ACS-PRF, Research Corporation, and the Camille and Henry Dreyfus Foundation. He has been named as a Project Kaleidoscope Faculty Member for the 21st Century and a Henry Dreyfus Teacher-Scholar.
Beyond his personal research efforts, Dr. Wheeler has directed Furman’s INBRE program since 2005 and developed Furman’s Office of Integrative Research in the Sciences in 2008, where he has served as the principal investigator (PI) for over $8.5M in programmatic awards from NIH-IDeA, NSF S-STEM, NSF RII, HHMI, Merck/AAAS, and the Sherman Fairchild Foundation. He and his wife, Dr. Sandy Wheeler, currently collaborate on research projects with faculty at Furman, Dr. Julia Brumaghim at Clemson University, and the Food and Drug Administration (FDA) in Silver Spring, Maryland.
Education
- Ph.D., University of Cincinnati
- B.S., Georgetown College
Research
Research in the Wheeler Group applies high-performance analytical techniques to a diverse series of chemical problems. Dr. Wheeler’s specialties are electrochemistry and separations science, but the group routinely uses spectroscopy, mass spectrometry, microcalorimetry and biochemical methods to address questions of clinical, environmental and forensic interest.

The group is currently active in three separate research areas: (1) investigating the ability of select compounds of sulfur and selenium to protect against reactive oxygen species (ROS) - induced DNA damage; (2) investigating DNA adduct formation and photocleavage activity with novel diimine complexes of Cr(III) in relation to photodynamic therapy; and (3) developing novel strategies for characterizing the mechanisms and properties of commercial biocide formulations:
- Oxidative DNA damage is one of the leading causes of cancer and other degenerative diseases, and Reactive Oxygen Species (ROS) (e.g., hydroxyl radical) are well associated with the formation of DNA lesions. One avenue through which ROS may be reduced and/or prevented is through the local availability of antioxidants. In collaboration with Dr. Julia Brumaghim at Clemson University, we are currently exploring the application of complexes of sulfur and selenium as potential antioxidants through mechanisms of action that have previously not been fully elucidated. The best know mechanism of DNA protection is radical scavenging, whereby an antioxidant reacts with ROS to form an unreactive product. In our work, however, we are investigating a mechanism of protection in which the target antioxidant (i.e., a S or Se-containing compound) may coordinate with a physiologically relevant metal of interest (e.g., Fe2+), thereby preventing the formation of ROS. As model systems for study, we are investigating compounds N,N’-dimethylimidazole thione (dmit) and selone (dmise), as well as methimazole (MetIm). Our results from gel electrophoresis and polymerase chain reaction (PCR) demonstrate that these three compounds protect DNA from oxidative damage, as evidenced by the continued successful replication of DNA after exposure to ROS-generating species such as hydrogen peroxide.
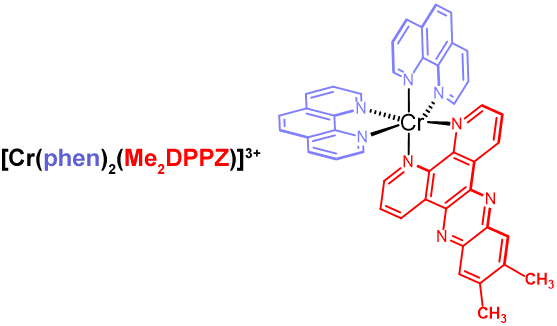
- In recent years we have collaborated with Dr. Noel Kane Maguire, Professor Emeritus, to establish that complexes of the type [Cr(diimine)2DPPZ]3+ (where DPPZ = dipyridophenazine) have the ability to intercalate with B-DNA, exhibiting significant binding affinities (KDNA > 105) and in many cases displaying unique enantioselectivities as a function of ligand identity. When excited by UV light, these complexes have thus demonstrated the potential to serve as chemotherapeutic agents, e.g., within the evolving field of photodynamic therapy, in which DNA may be cleaved owing to an electron transfer event between the 2Eg Cr(III) excited state and the nucleobase guanine. In some cases, monodentate ligands (i.e., 1-methylimidazole) have been substituted for one of the ancillary diimines, and following excitation by UV light, permanent DNA adduct formation has been observed. In our current work we are using a host of bioanalytical methodologies to investigate the nature, strength, and site of the Cr(III)/DNA interactions. This includes the application of isothermal titration calorimetry for studying binding thermodynamics (e.g., multiple DNA binding sites are observed), PCR and capillary electrophoresis for investigating the extent of DNA damage, and ultra high-performance liquid chromatography-electrospray mass spectrometry (UPLC-ESI-MS) for isolating and characterizing Cr(III)-DNA adducts and the products of photocleavage.

- Complex biocide formulations including agents such as polyhexamethyl biguanide (PHMB) are routinely used in multipurpose solutions (MPS) for contact lenses and wound dressings owing to their capacity to prevent bacterial infections. The concentrations of these agents required for efficacy are exceedingly small; in addition, the majority of these agents do not possess strong chromophores, and the combination of sample complexity and biocide polydispersity (e.g., PHMB) create a very challenging problem for separation and quantitation. We are currently working with the Food and Drug Administration (FDA) in Silver Spring, MD, to develop novel strategies for analysis of commercial MPS formulations, including studies of efficacy, adsorption to lens materials and estimates of human eye exposures using a variety of analytical strategies including solid phase extraction (SPE), dynamic light scattering (DLS), size-exclusion chromatography (SEC) and ultra pressure liquid chromatography – mass spectrometry (UPLC-MS) improving our understanding of how the structure of these biocides affects their biological efficacy is paramount in designing safe and effective contact lens treatments as well as other health care products for the future.
Honors and Awards
Beyond his personal research efforts, Dr. Wheeler has directed Furman’s INBRE program since 2005 and developed Furman’s Office of Integrative Research in the Sciences in 2008. Over this period he has served as the principal investigator (PI) for over $12.5M in programmatic awards from NIH-IDeA, NSF S-STEM, NSF RII, HHMI, Merck/AAAS, and the Sherman Fairchild Foundation supporting students faculty and staff research at Furman. He and his wife, Dr. Sandy Wheeler, currently collaborate on multiple research projects involving forensic, analytical and environmental chemistry and bioremediation.
-
Cass, A. L.; Slining, M. M.; Carson, C.; Cassidy, J.; Epright, M. C.; Gilchrist, A. E.; Peterson, K.; Wheeler, J. F.; The, S. T. Risk management of COVID-19 in the residential educational setting: lessons learned and implications for moving forward, Int J Environ Res Public Health, 2021, 18(18), 9743.
-
Bigner, J. A.; Fiester, S. E.; Fulcher, J. W.; Schammel, C. M. G.; Ward, M. E.; Burney, H. E.; Wheeler, J. F.; Wheeler, S. K.; Teuber, J. M. Glyphosate and polyoxyethyleneamine ingestion leading to renal, hepatic, and pulmonary failure, Am J Forensic Med Pathology, 2021, 42, 282-285.
-
Thompson, B. L.; Samant, V. N.; Wei, X.; David, F. D. C.; Edmunds, C. E.; Wheeler, J. M.; Phillips, K. S.; Wheeler, J. F.; Wheeler, S. K. Analysis of polyhexamethylene biguanide and alexidine in contact lens solutions using capillary electrophoresis, ultra-performance liquid chromatography and quadrupole time of flight mass spectrometry. Talanta, 2019, 205, 120056.
-
Goforth, S.K.; Gill, T.W.; Weisbruch, A.E.; Kane-Maguire, K.A.; Helsel, M.E.; Sun, K.W.; Rodgers, H.D.; Stanley, F.E.; Goudy, S.R.; Wheeler, S.K.; Wheeler, J.F.; Kane-Maguire, N.A.P. Synthesis of cis-[Cr(diimine)2(1-methylimidazole)2]3+ Complexes and an Investigation of Their Interaction with Mononucleotides and Polynucleotides. Inorg. Chem., 2016, 55(4), 1516-1526.
-
Petty, J. T.; Sergev, O. O.; Kantor, A. G.; Rankine, I. J.; Ganguly, M.; David, F. D.; Wheeler, S. K.; Wheeler, J. F. Ten-Atom Silver Cluster Signaling and Tempering DNA Hybridization. Anal. Chem. 2015, 87(10), 5302-5309.
-
Vandiver, M. S.; Bridges, E. P.; Koon, R. L.; Kinnaird, A. N.; Glaeser, J. W.; Campbell, J. F.; Priedemann, C. J.; Rosenblatt, W. T.; Herbert, B. J.; Wheeler, S. K.; Wheeler, J. F.; Kane-Maguire, N. Effect of ancillary ligands on the DNA interaction of Cr(diimine)3]3+ complexes containing the intercalating dipyridophenazine ligand. Inorg. Chem. 2010, 49, 839-48.
-
Kirchhoff, J. R.; Wheeler, J. F.; Lunte, C. E.; Jenkins, S. H.; Heineman, W. R. Electrochemistry: Principles and Measurement. In Clinical Chemistry: Theory Analysis and Correlation; Kaplan, L. A., Pesce, A. J., Eds.; C.V. Mosby Co., 2010.
-
Giardina, A.; Larson, S. E.; Wisner, B.; Wheeler, J. F.; Chao, M. Long-term and acute effects of zinc contamination of a stream on fish mortality and physiology. Environ. Toxicol. Chem. 2009, 28, 287-95.
-
Donnay, E. G.; Schaeper, J. P.; Brooksbank, R. D.; Fox, J. L.; Potts, R. G.; Davidson, R. M.; Wheeler, J. F.; Kane-Maguire, N. Synthesis and characterization of tris(heteroleptic) diimine complexes of chromium(III). Inorg. Chim. Acta 2007, 360, 3272-3280.
-
Herbert, B. J.; Carpenter, H. E.; Kane-Maguire, N.; Wheeler, J. F. Use of chiral capillary electrophoresis and circular dichroism for the determination of absolute values of Îε for diimine transition metal complexes. Anal. Chim. Acta 2004, 514, 27-35.
-
Harris, A. C.; Wheeler, J. F. GC-MS of ignitable liquids using solvent-desorbed SPME for automated analysis. J. Forensic Sci. 2003, 48, 41-46.
-
Schaeper, J. P.; Nelsen, L. A.; Shupe, M. A.; Herbert, B. J.; Kane-Maguire, N.; Wheeler, J. F. Capillary electrophoresis as a probe of enantiospecific interactions between photoactive transition metal complexes and DNA. Electrophoresis 2003, 24, 2704-10.
-
Andersen, C. B.; Sargent, K. A.; Wheeler, J. F.; Wheeler, S. K. Fluvial geochemistry of selected tributary watersheds in the Enoree River Basin, northwestern South Carolina. S. C. Geol. 2001, 43, 57-71.
-
Barker, K. D.; Barnett, K. A.; Connell, S. M.; Glaeser, J. W.; Wallace, A. J.; Wildsmith, J.; Herbert, B. J.; Wheeler, J. F.; Kane-Maguire, N. Synthesis and characterization of heteroleptic Cr(diimine)33+ complexes. Inorg. Chim. Acta 2001, 316, 41-49.
-
Barker, K. D.; Benoit, B. R.; Bordelon, J. A.; Davis, R. J.; Delmas, A. S.; Mytykh, O. V.; Petty, J. T.; Wheeler, J. F.; Kane-Maguire, N. Intercalative binding and photoredox behavior of Cr(phen)2(dppz)]3+ with B-DNA. Inorg. Chim. Acta 2001, 322, 74-78.
-
Harris, J. E.; Desai, N.; Seaver, K. E.; Watson, R. T.; Kane-Maguire, N.; Wheeler, J. F. Chiral separations of transition metal complexes using capillary zone electrophoresis. J. Chromatogr. A 2001, 919, 427-436.
-
Kane-Maguire, N.; Wheeler, J. F. Photoredox behavior and chiral discrimination of DNA bound M(diimine)3n+ complexes (M=Ru2+, Cr3+). Coord. Chem. Rev. 2001, 211, 145-162.
-
Koch, J. T.; Beam, B.; Phillips, K. S.; Wheeler, J. F. Hydrophobic interaction electrokinetic chromatography for the separation of polycyclic aromatic hydrocarbons using non-aqueous matrices. J. Chromatogr. A 2001, 914, 223-231.
-
Martin, S. E.; Maggie Connatser, R.; Kane-Maguire, N.; Wheeler, J. F. Capillary electrophoresis with laser-induced fluorescence detection for chiral analysis and DNA binding studies of ruthenium(II) Tris-diimine complexes. Anal. Chim. Acta 2001, 445, 21-27.
-
Mytykh, O. V.; Martin, S. E.; Wheeler, J. F.; Kane-Maguire, N. Application of capillary electrophoresis to stereochemical conversions. A case study: the photoracemization/hydrolysis of Cr(diimine)33+ species. Inorg. Chim. Acta 2000, 311, 143-146.
-
Watson, R. T.; Desai, N.; Wildsmith, J.; Wheeler, J. F.; Kane-Maguire, N. Interaction of Cr(diimine)3 3+ Complexes with DNA. Inorg. Chem. 1999, 38, 2683-2687.
-
Shelton, C. M.; Koch, J. T.; Desai, N.; Wheeler, J. F. Enhanced selectivity for capillary zone electrophoresis using ion-pair agents. J. Chromatogr. A 1997, 792, 455-462.
-
Shelton, C. M.; Seaver, K. E.; Wheeler, J. F.; Kane-Maguire, N. Application of Capillary Electrophoresis for the Assessment of Enantiomeric Purity of α-Diimine Transition Metal Complexes. Inorg. Chem. 1997, 36, 1532-1533.
-
Weldon, M. K.; Arrington, C. M.; Runnels, P. L.; Wheeler, J. F. Selectivity enhancement for free zone capillary electrophoresis using conventional ion-pairing agents as complexing additives. J. Chromatogr. A 1997, 758, 293-302.
-
Wheeler, J. F.; Wheeler, S. K.; Wright, L. L. Electrochemical Measurements in the Undergraduate Curriculum. J. Chem. Educ. 1997, 74, 72-72.