Sandy Wheeler
Senior Research Associate and Lecturer
- Email: sandy.wheeler@furman.edu
- Phone: 864.294.3377
- Office: Plyler 244A, Townes Science Center
Sandy Wheeler has served on the Chemistry faculty at Furman since 1992 after obtaining her Ph.D. from the University of Cincinnati. She began as Adjunct Professor while working as a consultant to establish a new environmental analysis company, and soon afterwards became the department’s Industrial Liaison to local companies. In the late 1990s, she increased her responsibilities to become the Instrumentation Specialist within the department.
In 2005, Furman was awarded an NIH-INBRE grant, and Dr. Wheeler further expanded her role to provide technical expertise in support of biomedical research efforts conducted in the Chemistry, Biology, and Psychology departments. The position included maintaining primary pieces of shared instrumentation used by INBRE target faculty (such as the LC-MS system housed in the Chemistry), faculty and student training, and method development and data acquisition. In 2015, Dr. Wheeler assumed her current title of Senior Research Associate and Lecturer, supported in part by an NIH award.
Working with her husband, John Wheeler, Associate Provost for Integrative Science, Dr. Wheeler co-supervises a highly active undergraduate effort at Furman, typically engaging up to a dozen science majors each year. In addition to her success in the classroom, her proudest professional accomplishments have been mentoring 120+ successful undergraduate students in Chemistry’s research program while maintaining and providing training of departmental instrumentation that serves her colleagues in Chemistry and across the university.
Education
- Ph.D.,University of Cincinnati
- B.S., University of Cincinnati
Research
Research in the Wheeler Group applies high performance analytical techniques to a diverse series of chemical problems. Dr. Wheeler’s group routinely uses spectroscopy, mass spectrometry, microcalorimetry and biochemical methods to address questions of clinical, environmental and forensic interest.

The group is currently active in three separate research areas: (1) investigating the ability of select compounds of sulfur and selenium to protect against reactive oxygen species (ROS) -induced DNA damage; (2) investigating DNA adduct formation and photocleavage activity with novel diimine complexes of Cr(III) in relation to photodynamic therapy; and (3) developing novel strategies for characterizing the mechanisms and properties of commercial biocide formulations:
-
- Oxidative DNA damage is one of the leading causes of cancer and other degenerative diseases, and Reactive Oxygen Species (ROS) (e.g., hydroxyl radical) are well associated with the formation of DNA lesions. One avenue through which ROS may be reduced and/or prevented is through the local availability of antioxidants. In collaboration with Dr. Julia Brumaghim at Clemson University, we are currently exploring the application of complexes of sulfur and selenium as potential antioxidants through mechanisms of action that have previously not been fully elucidated. The best know mechanism of DNA protection is radical scavenging, whereby an antioxidant reacts with ROS to form an unreactive product. In our work, however, we are investigating a mechanism of protection in which the target antioxidant (i.e., a S or Se-containing compound) may coordinate with a physiologically relevant metal of interest (e.g., Fe2+), thereby preventing the formation of ROS. As model systems for study, we are investigating compounds N,N’-dimethylimidazole thione (dmit) and selone (dmise), as well as methimazole (MetIm). Our results from gel electrophoresis and polymerase chain reaction (PCR) demonstrate that these three compounds protect DNA from oxidative damage, as evidenced by the continued successful replication of DNA after exposure to ROS-generating species such as hydrogen peroxide.
-
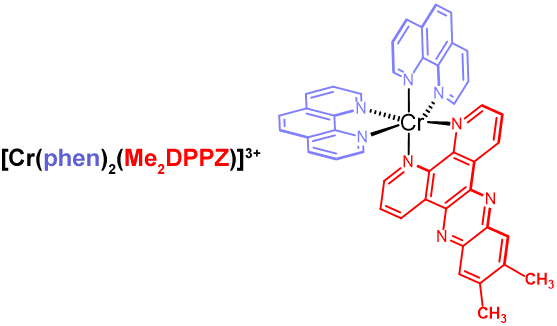
- In recent years we have collaborated with Dr. Noel Kane Maguire, Professor Emeritus, to establish that complexes of the type [Cr(diimine)2DPPZ]3+ (where DPPZ = dipyridophenazine) have the ability to intercalate with B-DNA, exhibiting significant binding affinities (KDNA > 105) and in many cases displaying unique enantioselectivities as a function of ligand identity. When excited by UV light, these complexes have thus demonstrated the potential to serve as chemotherapeutic agents, e.g., within the evolving field of photodynamic therapy, in which DNA may be cleaved owing to an electron transfer event between the 2Eg Cr(III) excited state and the nucleobase guanine. In some cases, monodentate ligands (i.e., 1-methylimidazole) have been substituted for one of the ancillary diimines, and following excitation by UV light, permanent DNA adduct formation has been observed. In our current work we are using a host of bioanalytical methodologies to investigate the nature, strength, and site of the Cr(III)/DNA interactions. This includes the application of isothermal titration calorimetry for studying binding thermodynamics (e.g., multiple DNA binding sites are observed), PCR and capillary electrophoresis for investigating the extent of DNA damage, and ultra high-performance liquid chromatography-electrospray mass spectrometry (UPLC-ESI-MS) for isolating and characterizing Cr(III)-DNA adducts and the products of photocleavage.

- In recent years we have collaborated with Dr. Noel Kane Maguire, Professor Emeritus, to establish that complexes of the type [Cr(diimine)2DPPZ]3+ (where DPPZ = dipyridophenazine) have the ability to intercalate with B-DNA, exhibiting significant binding affinities (KDNA > 105) and in many cases displaying unique enantioselectivities as a function of ligand identity. When excited by UV light, these complexes have thus demonstrated the potential to serve as chemotherapeutic agents, e.g., within the evolving field of photodynamic therapy, in which DNA may be cleaved owing to an electron transfer event between the 2Eg Cr(III) excited state and the nucleobase guanine. In some cases, monodentate ligands (i.e., 1-methylimidazole) have been substituted for one of the ancillary diimines, and following excitation by UV light, permanent DNA adduct formation has been observed. In our current work we are using a host of bioanalytical methodologies to investigate the nature, strength, and site of the Cr(III)/DNA interactions. This includes the application of isothermal titration calorimetry for studying binding thermodynamics (e.g., multiple DNA binding sites are observed), PCR and capillary electrophoresis for investigating the extent of DNA damage, and ultra high-performance liquid chromatography-electrospray mass spectrometry (UPLC-ESI-MS) for isolating and characterizing Cr(III)-DNA adducts and the products of photocleavage.
- Complex biocide formulations including agents such as polyhexamethyl biguanide (PHMB) are routinely used in multipurpose solutions (MPS) for contact lenses and wound dressings owing to their capacity to prevent bacterial infections. The concentrations of these agents required for efficacy are exceedingly small; in addition, the majority of these agents do not possess strong chromophores, and the combination of sample complexity and biocide polydispersity (e.g., PHMB) create a very challenging problem for separation and quantitation. We are currently working with the Food and Drug Administration (FDA) in Silver Spring, MD, to develop novel strategies for analysis of commercial MPS formulations, including studies of efficacy, adsorption to lens materials and estimates of human eye exposures using a variety of analytical strategies including solid phase extraction (SPE), dynamic light scattering (DLS), size-exclusion chromatography (SEC) and ultra pressure liquid chromatography – mass spectrometry (UPLC-MS) improving our understanding of how the structure of these biocides affects their biological efficacy is paramount in designing safe and effective contact lens treatments as well as other health care products for the future.
-
Thompson, B. L.; Samant, V. N.; Wei, X.; David, F. D. C.; Edmunds, C. E.; Wheeler, J. M.; Phillips, K. S.; Wheeler, J. F.; Wheeler, S. K. Analysis of polyhexamethylene biguanide and alexidine in contact lens solutions using capillary electrophoresis, ultra-performance liquid chromatography and quadrupole time of flight mass spectrometry. Talanta, 2019, 205, 120056.
-
Goforth, S.K.; Gill, T.W.; Weisbruch, A.E.; Kane-Maguire, K.A.; Helsel, M.E.; Sun, K.W.; Rodgers, H.D.; Stanley, F.E.; Goudy, S.R.; Wheeler, S.K.; Wheeler, J.F.; Kane-Maguire, N.A.P. Synthesis of cis-[Cr(diimine)2(1-methylimidazole)2]3+ Complexes and an Investigation of Their Interaction with Mononucleotides and Polynucleotides. Inorg. Chem., 2016, 55(4), 1516-1526.
-
Petty, J. T.; Sergev, O. O.; Kantor, A. G.; Rankine, I. J.; Ganguly, M.; David, F. D.; Wheeler, S. K.; Wheeler, J. F. Ten-Atom Silver Cluster Signaling and Tempering DNA Hybridization. Anal. Chem. 2015, 87(10), 5302-5309.
-
Vandiver, M. S.; Bridges, E. P.; Koon, R. L.; Kinnaird, A. N.; Glaeser, J. W.; Campbell, J. F.; Priedemann, C. J.; Rosenblatt, W. T.; Herbert, B. J.; Wheeler, S. K.; Wheeler, J. F.; Kane-Maguire, N. Effect of ancillary ligands on the DNA interaction of Cr(diimine)3]3+ complexes containing the intercalating dipyridophenazine ligand. Inorg. Chem. 2010, 49, 839-48.
-
Andersen, C. B.; Sargent, K. A.; Wheeler, J. F.; Wheeler, S. K. Fluvial geochemistry of selected tributary watersheds in the Enoree River Basin, northwestern South Carolina. S. C. Geol. 2001, 43, 57-71.
-
Wheeler, J. F.; Wheeler, S. K.; Wright, L. L. Electrochemical Measurements in the Undergraduate Curriculum. J. Chem. Educ. 1997, 74, 72-72.